Research
Overview
Our group applies mechanistic principles to develop new concepts in catalysis. Our goal is to advance new conceptual strategies for catalytic reactions to provide novel solutions to problems ranging from materials design, energy conversion, selective oxidation reactions, to drug delivery. Areas of focus include the development of organic or organometallic catalysts for the synthesis of macromolecules with novel chemical, physical or biological properties, selective catalytic (including electrocatalytic) redox reactions, and the application of new in-situ techniques for interrogating catalytic reactions.
- Organocatalytic Polymerization Reactions
- Functional Biodegradable Polymers for Biomedical Applications
- Electrocatalysis for Energy-Efficient Fuel Conversion
- Sustainable Materials for the 21st Century
Organocatalytic Polymerization Reactions

Our group has pioneered the design and application of organic catalysts for polymer chemistry. In collaboration with Dr. James Hedrick of IBM, we have developed a platform of organic catalysts that exhibit activities that rival the most active metal-based catalysts and, by virtue of their novel mechanisms of enchainment, provide access to polymer architectures that are difficult to access by conventional approaches. Significant achievements include organocatalytic strategies for the synthesis of polyesters, polycarbonates, polysiloxanes, and polyacrylates, the chemical recycling of commodity polyesters, and the generation of new families of biocompatible polymers for biomedical applications. The development of new families of organic polymerization catalysts have stimulated worldwide interest in this strategy for the generation of new families of biodegradable materials.
Morodo, R.; Dumas, D.M.; Zhang, J.; Waymouth, R.M.; Hedrick, J.L. “Ring-Opening Polymerization of Cyclic Esters and Carbonates with (Thio)urea/Cyclopropenimine Organocatalytic Systems” ACS Macro Lett. 2024, 13, 181-188. doi: 10.1021/acsmacrolett.3c00716
Zhang, J.; Lui, K.H.; Hedrick, J.L.; Waymouth, R.M. “Contrasting Roles of Counterions in Anionic Ring-Opening Polymerization Mediated by Heterocycle Organocatalysts” ACS Catal. 2023, 13(24), 16097-16104, doi: 10.1021/acscatal.3c04772.
Jadrich, C.N.; Pane, V.E.; Park, N.H.; Waymouth, R.M. “A Cation-Dependent Dual Activation Motif for Anionic Ring-Opening Polymerization of Cyclic Esters” J. Am. Chem. Soc. 2022, 144(19) 8439–8443. doi: 10.1021/jacs.2c01436
Lin, B.; Hedrick, J. L.; Park, N. H.; Waymouth, R. M. “Programmable High-Throughput Platform for the Rapid and Scalable Synthesis of Polyester and Polycarbonate Libraries” J. Am. Chem. Soc. 2019, 141(22), 8921-8927, doi: 10.1021/jacs.9b02450.
Lin, B.; Waymouth, R. M. “Organic Ring-Opening Polymerization Catalysts: Reactivity Control by Balancing Acidity” Macromolecules, 2018, 51(8), 2932–2938, doi: 10.1021/acs.macromol.8b00540.
Lin, B.; Waymouth, R. M. “Urea Anions: Simple, Fast, and Selective Catalysts for Ring-Opening Polymerizations” J. Am. Chem. Soc., 2017, 139(4), 1645–1652, doi: 10.1021/jacs.6b11864.
Functional Biodegradable Polymers for Biomedical Applications

We have exploited the high functional tolerance of organic catalysts to generate several families of biodegradable functional polyesters and polycarbonates for biomedical applications. In collaboration with the Wender group, we have generated a family of functionalized polymers that mimic the function of cell-penetrating peptides for the delivery of drugs and probes and nucleic acids into cells, as well as injectable hydrogels and self-assembled nanoparticles for drug delivery. We have discovered a general, safe, and remarkably effective concept for RNA delivery based on a new class of synthetic cationic materials, Charge-Altering Releasable Transporters (CARTs). These new materials operate by an unprecedented mechanism for the delivery and transcription of proteins in both cell culture and live animals. The CARTs behave as “physical property chameleons” changing from polycations that complex and protect RNA, to neutral species that release RNA upon cell entry.
Li, Z.; Amaya, L.; Wang, S. K.; Ranjan, A.; Waymouth, R. M.; Chang, H. Y.; Wender, P. A. ” Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism”, Nat. Commun. 2023, 14, 6983. doi: 10.1038/s41467-023-42672-x
Blake, T. R.; Haabeth, O. A. W.; Sallets, A.; McClellan. R. L.; Del Castillo, T. J.; Viches-Moure, J. G.; Ho, W. C.; Wender, P.A.; Levy, R.; Waymouth, R. M. “Lysine-derived Charge-Altering Releasable Transporters (K-CARTs): Targeted Delivery of mRNA and siRNA to the Lungs” ACS Bioconjugate Chem., 2023, 34, 673-685. doi: 10.1021/acs.bioconjchem.3c00019.
Haabeth, O. A. W.; Lohmeyer, J. J. K.; Sallets, A.; Blake, T. R.; Sagiv-Barfi, I.; Czerwinski, D. K.; Powell, A. E.; Wender, P. A.; Waymouth, R. M.; Levy, R., “An mRNA SARS-CoV-2 Vaccine Employing Charge-Altering Releasable Transporters with a TLR-9 Agonist Induces Neutralizing Antibodies and T Cell Memory” ACS Central Science., 2021, 7(7), 1191-1204. doi: 10.1021/acscentsci.1c00361
Haalbeth, O.; Blake, T.; McKinlay, C. J.; Waymouth, R. M.; Wender, P. A.; Levy, R.; “mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice” Proc. Nat. Acad. Sci., 2018, 115(39) E9153-E9161. doi: 10.1073/pnas.1810002115
McKinlay, C.J.; Benner, N.L.; Haabeth, O.A.; Waymouth, R. M.; Wender, P.A. “Enhanced mRNA delivery into lymphocytes enable by lipid-varied libraries of Charge-Altering Releasable Transporters” Proc. Nat. Acad. Sci., 2018, 115(26), E5859-E5866. doi: 10.1073/pnas.1805358115.
Electrocatalysis for Energy-Efficient Fuel Conversion
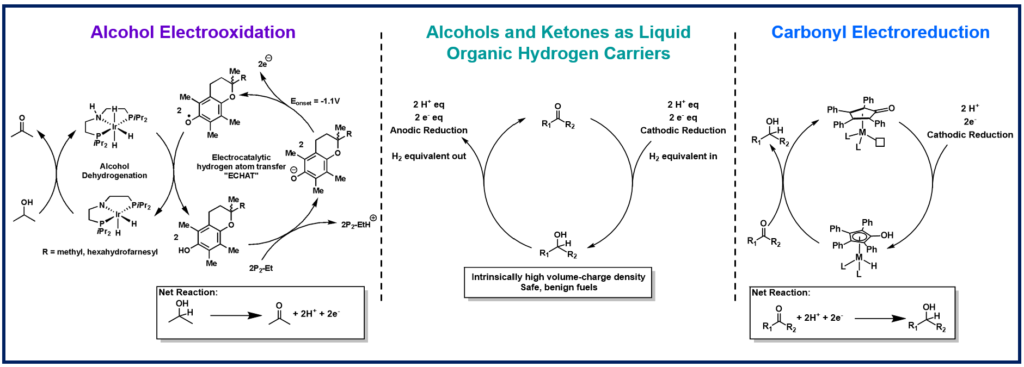
Grid scale storage of renewable energy is necessary to modernize our energy production and cease use of fossil fuels. Liquid organic hydrogen carriers (LOHCs), such as alcohols, are attractive energy carriers. Our goal is to develop electrocatalytic strategies for alcohol oxidation and ketone reduction to illuminate and define the scientific opportunities and challenges for developing electrochemically regenerable LOHCs for energy storage.
We are investigating highly active transfer hydrogenation catalysts as potential electrocatalysts. Metal hydrides are key intermediates in transfer hydrogenations, but the electrochemical generation and oxidation of metal hydrides are energetically inefficient as they typically require multiple stepwise proton and electron transfers. We have discovered several electrocatalytic mediators to electrochemically generate and oxidize metal hydrides by concerted proton and electron transfer (CPET) to bypass the high energy, stepwise pathways. These mediated strategies can enable efficient electrooxidation of alcohols and electroreduction of ketones.
Galvin, C.M.; Marron, D.P.; Dressel, J.M.; Waymouth, R.M.; “Coordination-Induced Bond Weakening and Electrocatalytic Proton-Coupled Electron Transfer of a Ruthenium Verdazyl Complex”, Inorg. Chem. 2024, 63(2) 954-960. doi: 10.1021/acs.inorgchem.3c02775
Marron, D.P.; Galvin, C.M.; Waymouth, R.M.; “Cyclopentadienone Iridium Bipyridyl Complexes: Acid-Stable Transfer Hydrogenation Catalysts”, Organometallics. 2023, 42(15), 1849-1853. doi: 10.1021/acs.organomet.3c00266
Galvin, C.; Waymouth, R.M.; “Electron-rich phenoxyl mediators improve thermodynamic performance of electrocatalytic alcohol oxidation with an iridium pincer complex”, J. Am. Chem. Soc. 2020, 142(45), 19368-19378. doi: 10.1021/jacs.0c09605.
McLoughlin, E.A.; Armstrong, K.; Waymouth, R.M.; “Electrochemically regenerable hydrogen atom acceptors: mediators in electrocatalytic alcohol oxidation reactions”, ACS Catal., 2020, 10(19), 11654-11662. doi: 10.1021/acscatal.0c03240.
McLoughlin, E. A.; Giles, L. J.; Waymouth, R. M.; Sarangi, R.; “X-ray Absorption Spectroscopy and Theoretical Investigation of the Reductive Protonation of Cyclopentadienyl Cobalt Compounds”, Inorg. Chem., 2019, 58, 1167-1176. doi: 10.1021/acs.inorgchem.8b02475
Matson, B. D.; McLoughlin, E. A.; Armstrong, K.; Waymouth, R. M.; Sarangi, R. “Effect of Redox-Active Ligands on the Electrochemical Properties of Manganese Tricarbonyl Complexes” Inorg. Chem. 2019, 58.7453-7465. doi: 10.1021/acs.inorgchem.9b00652
Sustainable Materials for the 21st Century
We are developing catalytic strategies for generating sustainable materials for the 21st century, where materials are designed not only for function and performance, but incorporate into their structural design means for recovery and reuse. In collaboration with Jim Hedrick of IBM, Matthew Kanan of Stanford and Craig Criddle of Stanford, we have developed chemical and microbial catalytic chemical recycling strategies for plastics, and new strategies for generating plastics from non-traditional resources, including waste streams, CO2 and inedible biomass.
Blake, T. R.; Ho, W. C.; Turlington, C.R.; Zhang, X.; Huttner, M. A.; Wender, P. A.; Waymouth, R. M. “Synthesis and Mechanistic Investigations of pH-responsive cationic poly(aminoester)s” Chem. Sci., 2020, 11, 2951-2966. doi: 10.1039/c9sc05267d
Zhang, X.; Fevre, M.; Jones, G. O.; Waymouth, R. M. “Catalysis as an Enabling Science for Sustainable Polymers” Chem. Rev., 2018, 118, 839-885. doi: 10.1021/acs.chemrev.7b00329.
Fukushima, K.; Coulembier, O.; Lecuyer, J. M.; Al-Megren, H. A.; Mohammad, A.; Alsewailem, F. D.; McNeil, M. A.; Dubois, P.; Waymouth, R. M.; Horn, H. W.; Rice, J. E.; Hedrick, J. L. “Closing the Loop on Recycling: Organocatalytic Depolymerization of Poly(ethyleneterephthalate)” J. Polym. Sci. Part A: Polym. Chem., 2011 49, 1273-1281. doi: 10.1002/pola.24551.
Funding
We are grateful for the generous support of several funding agencies:
The National Science Foundation under Grant Nos. CHE-2002933, CHE-2101256; the National Institute of Health R01 CA245533, The Wellcome Leap Foundation, the Stanford Precourt Institute for Energy, the Stanford Woods Institute for the Environment, the SPARK Translational Research Program in the Stanford University School of Medicine.
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation, the National Institute of Health or Stanford University.